Question
According to Mendeleev’s Periodic law, the elements
were arranged in the periodic table in the order of…………...?Solution
The Mendeleev's periodic law is an arrangement of elements in increasing order of their atomic masses as Mendeleev's periodic law states that, the physical and chemical properties of elements are a periodic function of their atomic masses.
Which of the following is an input device?
Which of the following States is not covered under Strengthening Teaching-Learning and Results for States (STARS) Project?
The BSE (Bombay Stock Exchange) is designed to reflect the growth and performance of the top listed companies. How many companies are included in the BS...
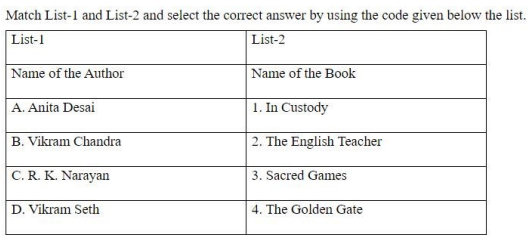
Which of the following is an auxiliary river of the Ganges originates from the high peninsular land?
Match the following Prime Ministers of India with the Five-Year Plans they initiated.
Which British scientist is known for developing the principle of holography in 1947 to improve the resolution of the electron microscope?
Recently in March the PF interest rate on EPF reduced by how much percent?
Asad Ali Khan was known for playing which of the following Indian instruments?
Which of the following options accurately describes the focus of the World Bank's and IMF's respective reports?
Relevant for Exams:



