Question
Isobars
are:Solution
Isobars have the same mass number but differ in the number of protons, resulting in different atomic numbers.
Statement: A ≤ D < C = B > E ≥ F < G
Conclusions: I. B > A
II. C < F
Which of the following letter-clusters will replace the question mark (?) in the given series?
RI, YB, FU, MN, TG, ?
Select the option that is related to the third word in the same way as the second word is related to the first word. (The words must be considered as me...
‘A × B’ means ‘A is B’s brother’.
‘A ÷ B’ means ‘A is B’s sister’.
‘A + B’ means ‘A is B’s father’.
Which of the following numbers will replace the question mark (?) in the given series?
304, 261, 221, 184, ?, 119
Select the correct mirror image of the given figure, when the mirror is placed at line MN as shown.
‘A @ B’ means ‘A is the wife of B’
‘A = B’ means ‘A is the brother of B’
‘A # B’ means ‘A is the son of B’
...
72 is related to 96 following a certain logic. Following the same logic, 66 is related to 88. Which of the following numbers is related to 104 using the...
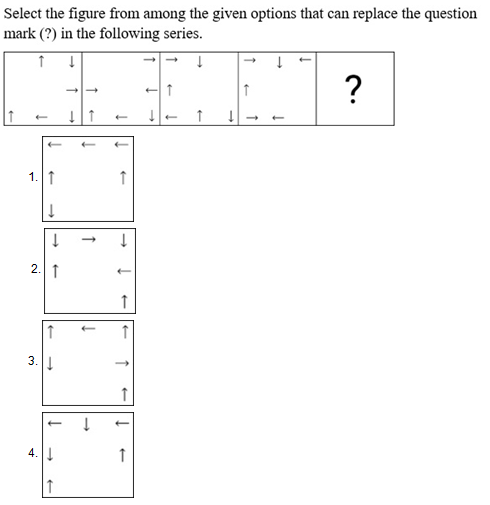
Select the set in which the numbers are related in the same way as are the numbers of the following sets.
(NOTE: Operations should be performed o...



